Selon le modèle de Michaelis et Menten, l'équation décrivant la vitesse initiale stationnaire d'une réaction enzymatique est la suivante : Avec : v i {\displaystyle v_ {i}} : vitesse initiale (c'est-à-dire en absence de produit) stationnaire (indépendante du temps) de la réaction enzymatique pour une concentration initiale en substrat.. The Michaelis-Menten kinetics equation includes two key terms: Maximum reaction rate, or V max, occurs when all substrate binding sites in an enzyme are full. Michaelis constant or K m, is the concentration of substrate at which the reaction rate is half of its maximum value (V max).The K m value indicates how well an enzyme is able to perform its activity at different concentrations of its.

12. Cinétique MichaelisMenten. La formation du complexe {ES} est... Download Scientific Diagram
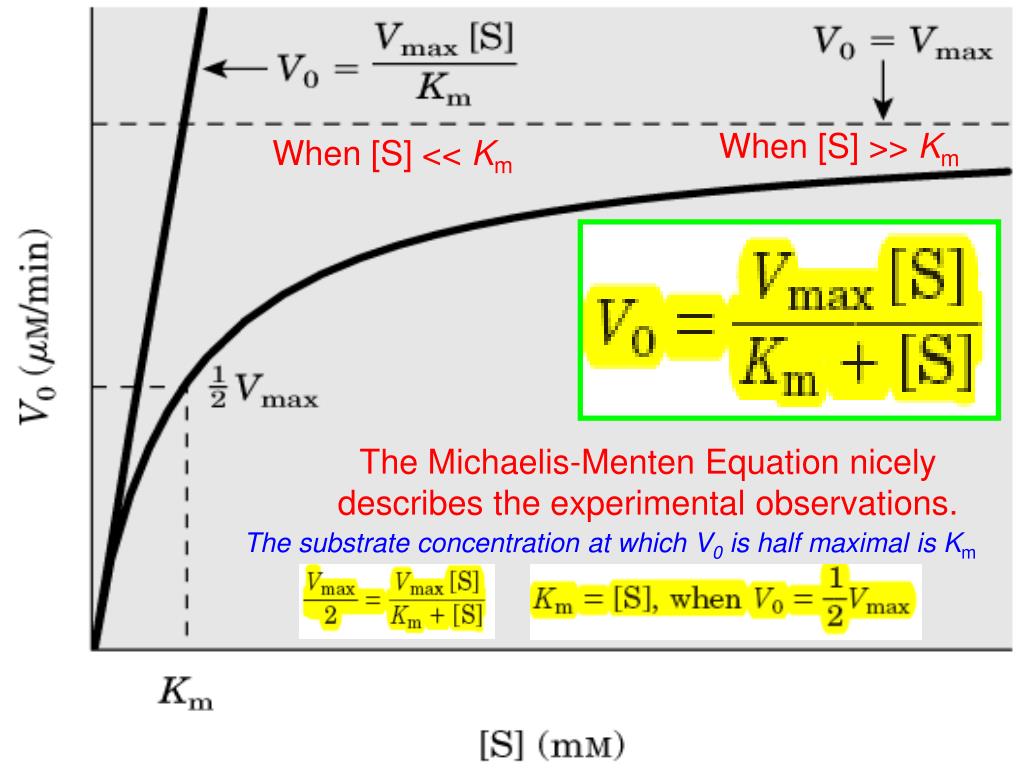
PPT The MichaelisMenten Equation nicely describes the experimental observations. PowerPoint
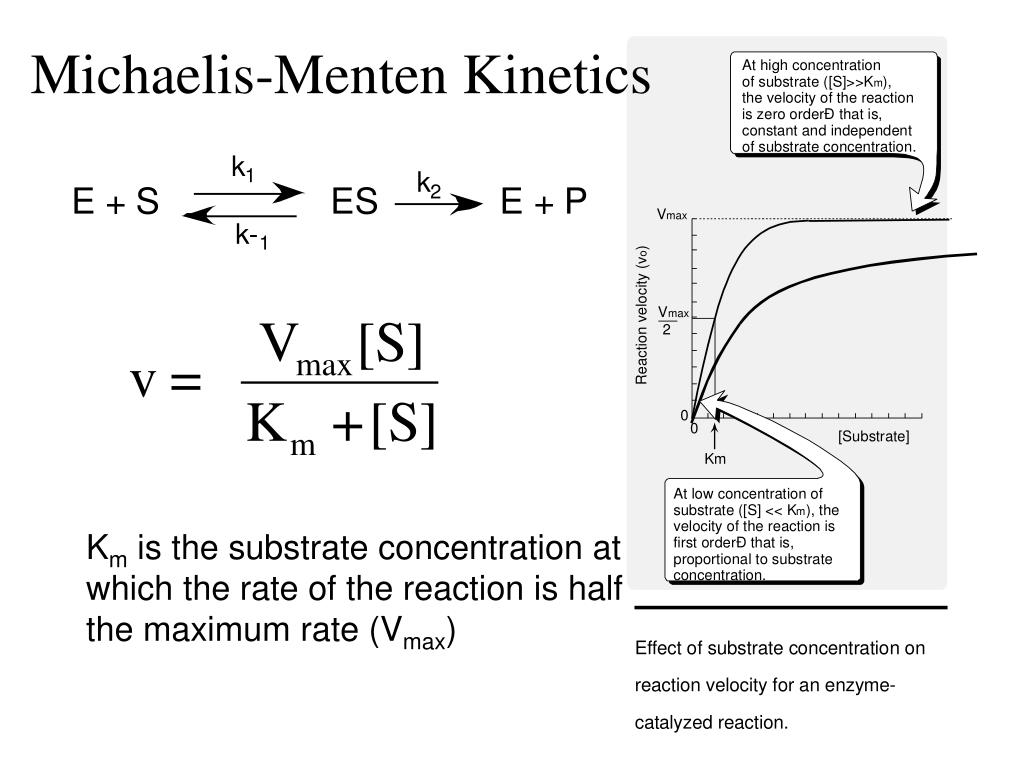
PPT MichaelisMenten PowerPoint Presentation, free download ID6844173
.png?revision=1&size=bestfit&width=571&height=383)
7.2 Derivation of MichaelisMenten equation (2022)
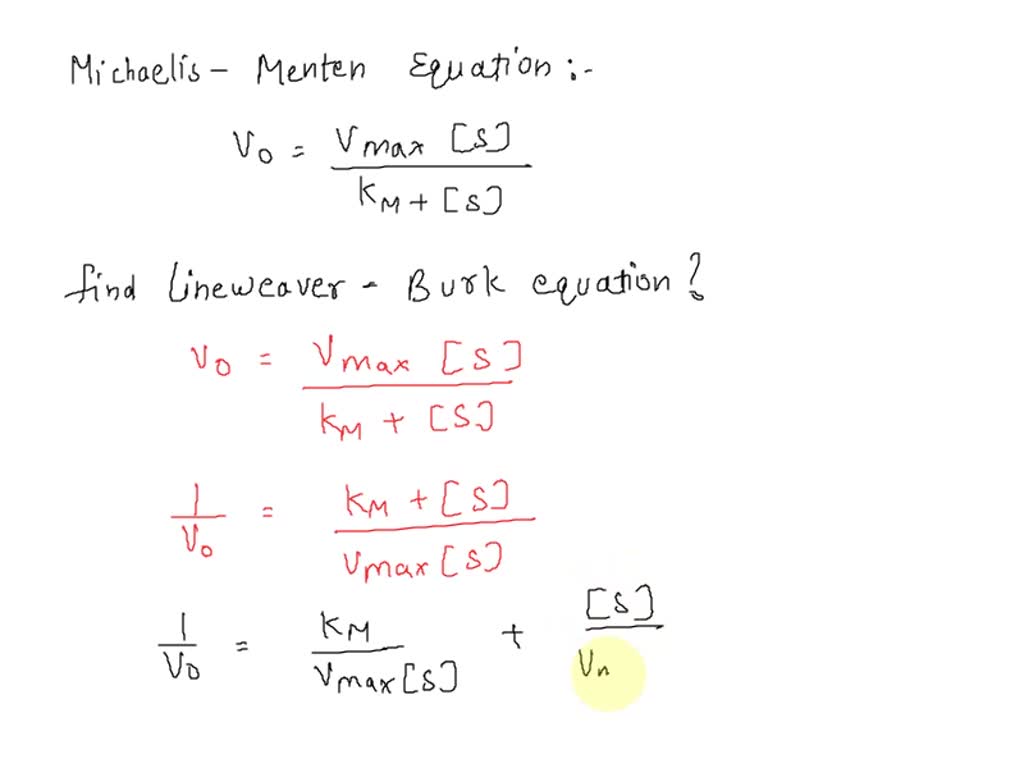
SOLVED Derive the LineweaverBurk equation from the MichaelisMenten equation (WRITE DOWN ALL

MichaelisMenten Equation Enzyme Biochemistry, Science resources

MichaelisMenten equation Interactive graph PhysiologyWeb
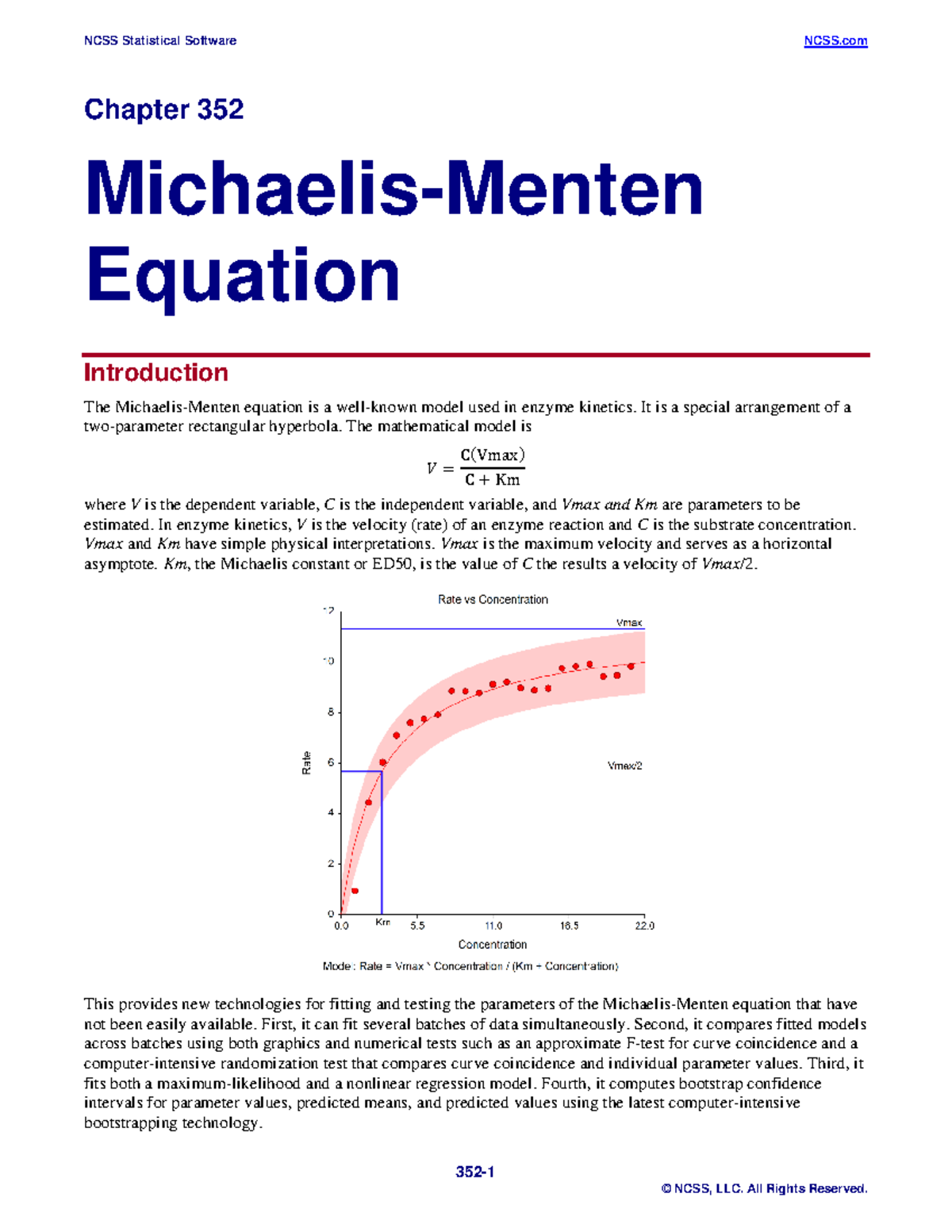
MichaelisMenten Equation It is a special arrangement of a twoparameter rectangular hyperbola

Km Michaelis Menten Equation Enzyme YouTube
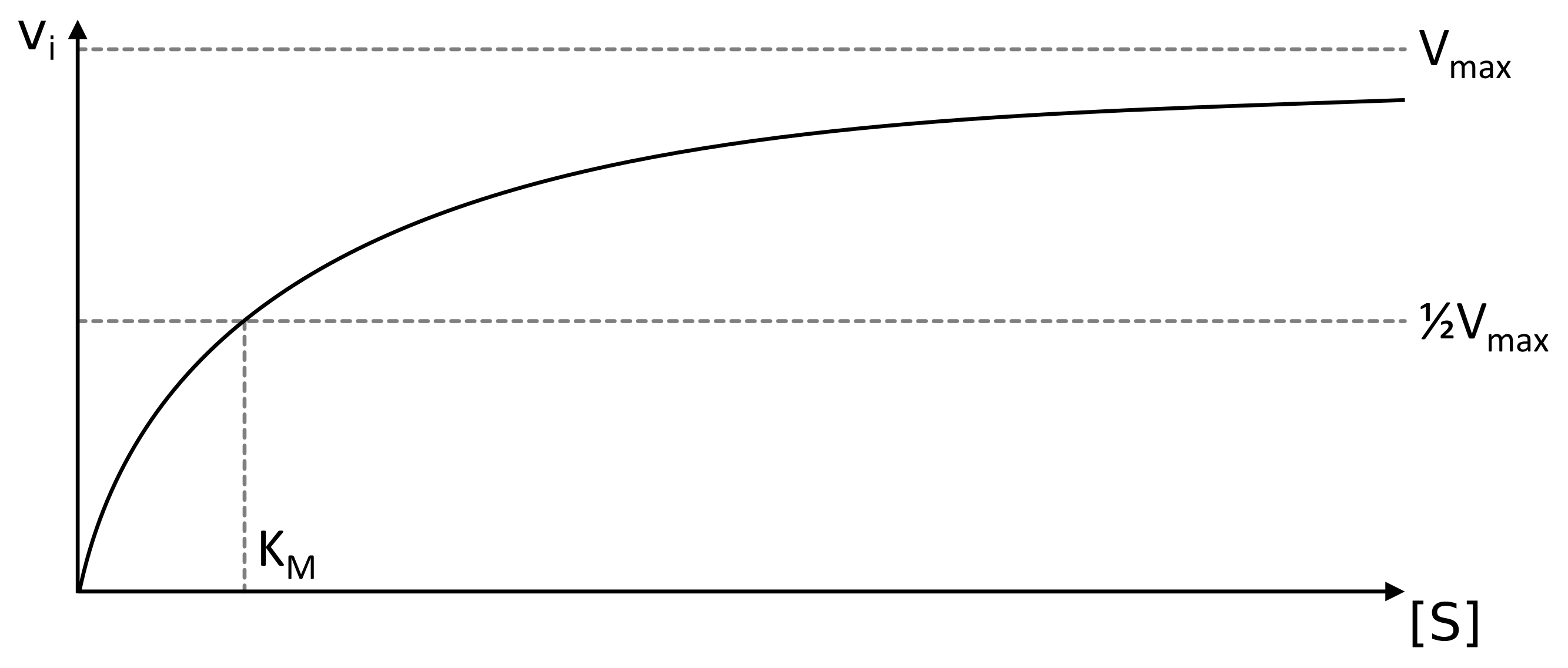
La cinétique des enzymes michaeliennes et l'équation de MichaelisMenten

SOLUTION Lineweaver burk eadie hofstee hanes plot derivation from michaelis menten equation
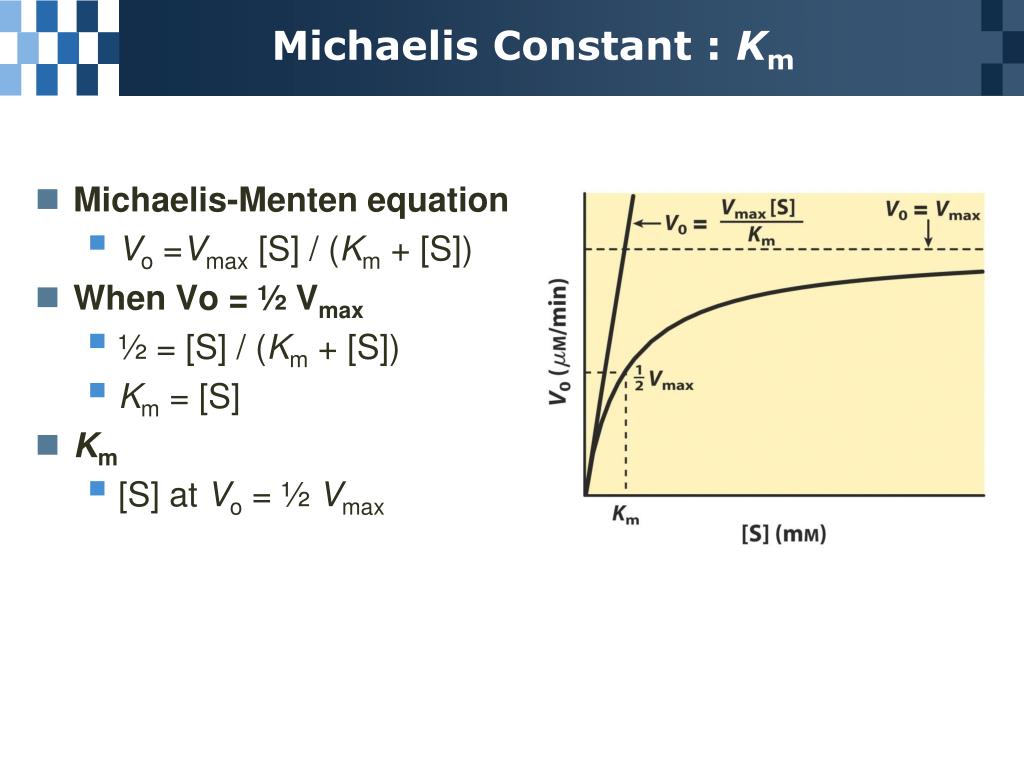
PPT Chapter 6 Enzyme PowerPoint Presentation, free download ID5692414

4.2a Derivation of the MichaelisMenten Equation Chad's Prep®

Developing a three‐dimensional animation for deeper molecular understanding of michaelismenten

PPT The MichaelisMenten Equation nicely describes the experimental observations. PowerPoint

Image Gallery Michaelismenten Curve

II 2 2 Le modèle de Michaelis Menten YouTube
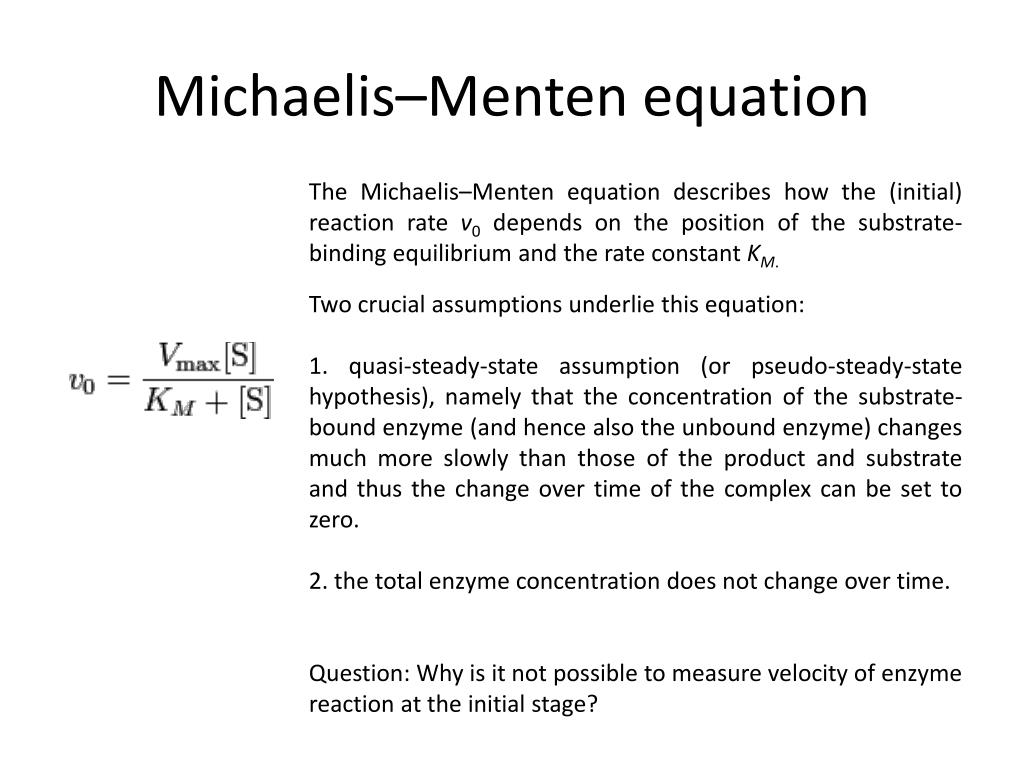
PPT Lecture 4 PowerPoint Presentation, free download ID2832807

044MichaelisMenten Equation YouTube

Michaelis Menten Equation Enzyme (PART 1) Introduction YouTube
Michaelis and Menten equation (MM equation) has dominated biochemistry for more than a century after its seminal introduction in a paper published in 1913 in the journal Biochemische Zeitschrift, a predecessor of FEBS Journal [[]].Hence, publishing this guide in FEBS Journal would represent an apt dedication to the unmatched service rendered by this journal to the scientific community for more.. The Michaelis-Menten analysis incorporates the additional assumption that k 2 is much less than k-1,. A. R. Fersht, 1988, pp98-101. The rate equation can be integrated in two ways: Approximation I: Re-introducing the assumption that [E] is small, set [A] = [A] 0 - [C]. The Briggs-Haldane equation can now be integrated: