About 'Acid + Metal Oxide' Reactions. Acid + Metal Oxide reactions are exothermic so the there will be a temperature rise. Acid + Metal Oxide reactions occur when the Metal in the Metal Oxide is more reactive than Hydrogen. This means the metal can lose electrons more easily than Hydrogen can. During an Acid + Metal Oxide reaction the H + ions.. 14.5: Reactions of Acids and Bases is shared under a Public Domain license and was authored, remixed, and/or curated by Marisa Alviar-Agnew, Henry Agnew, Peggy Lawson, & Peggy Lawson. When an acid and a base are combined, water and a salt are the products. Salts are ionic compounds containing a positive ion other than H+ and a negative ion.

Reaction of Metals and NonMetals with Acids Teachoo Concepts

PPT How are salts made and named? PowerPoint Presentation, free download ID5348558
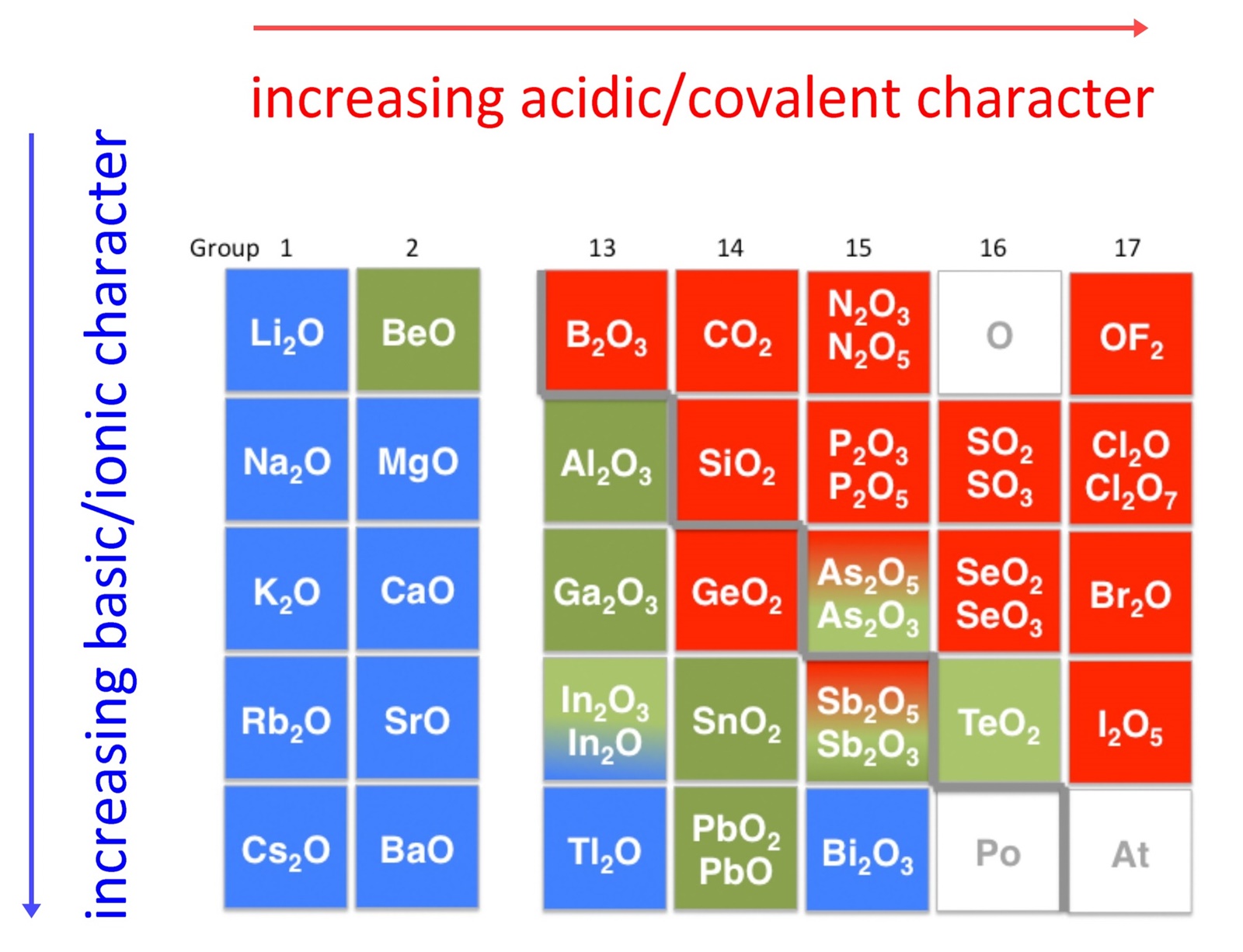
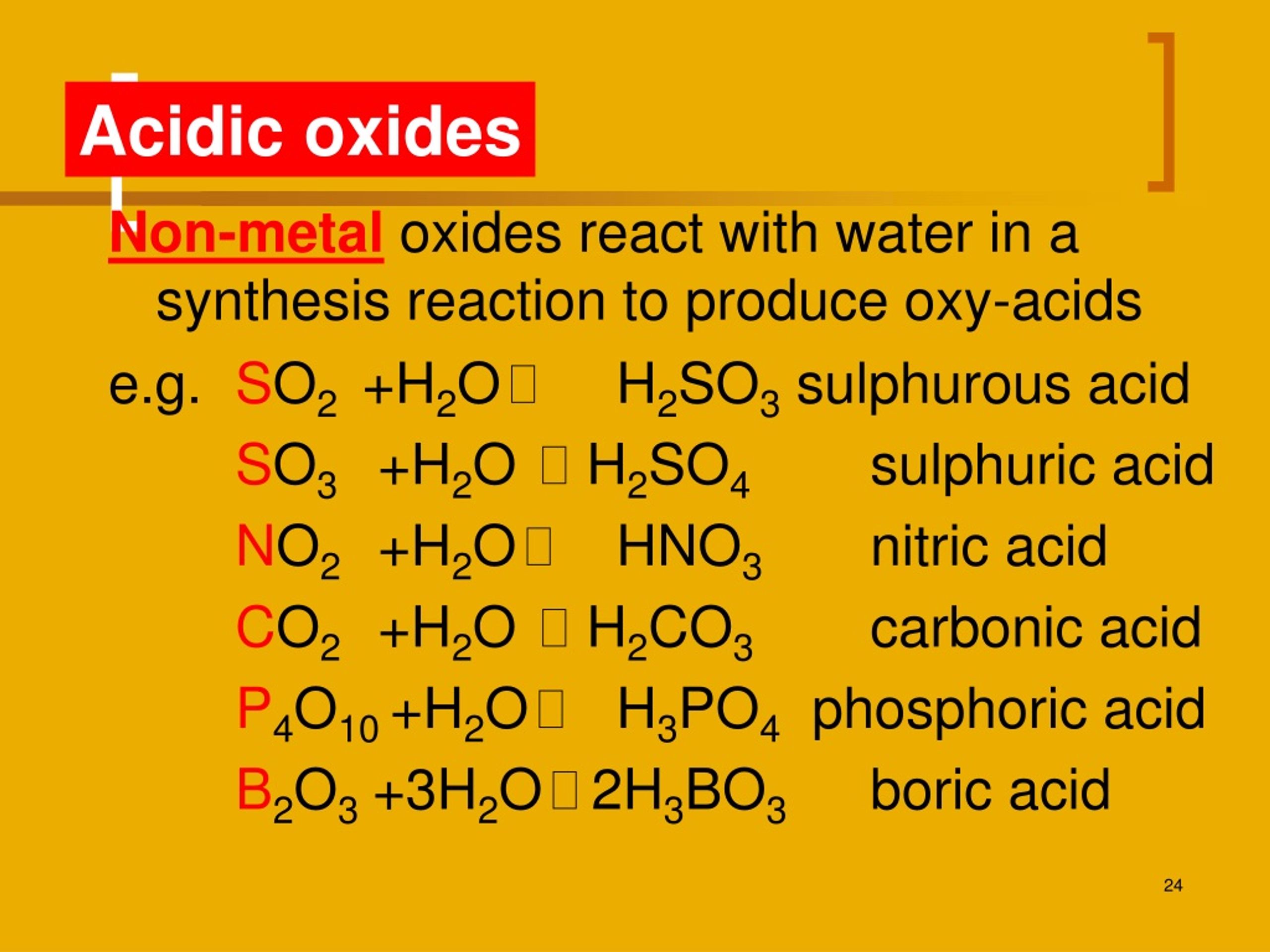
Examples of Acidic Oxides KaraaddFarrell

Metal And Acid Reaction How acids react with metal carbonates CBSE Class 10 / Here are
Reaction of Metal Oxides with Acids and Nonmetal oxides with Bases Class X (Chap 2 Part XI

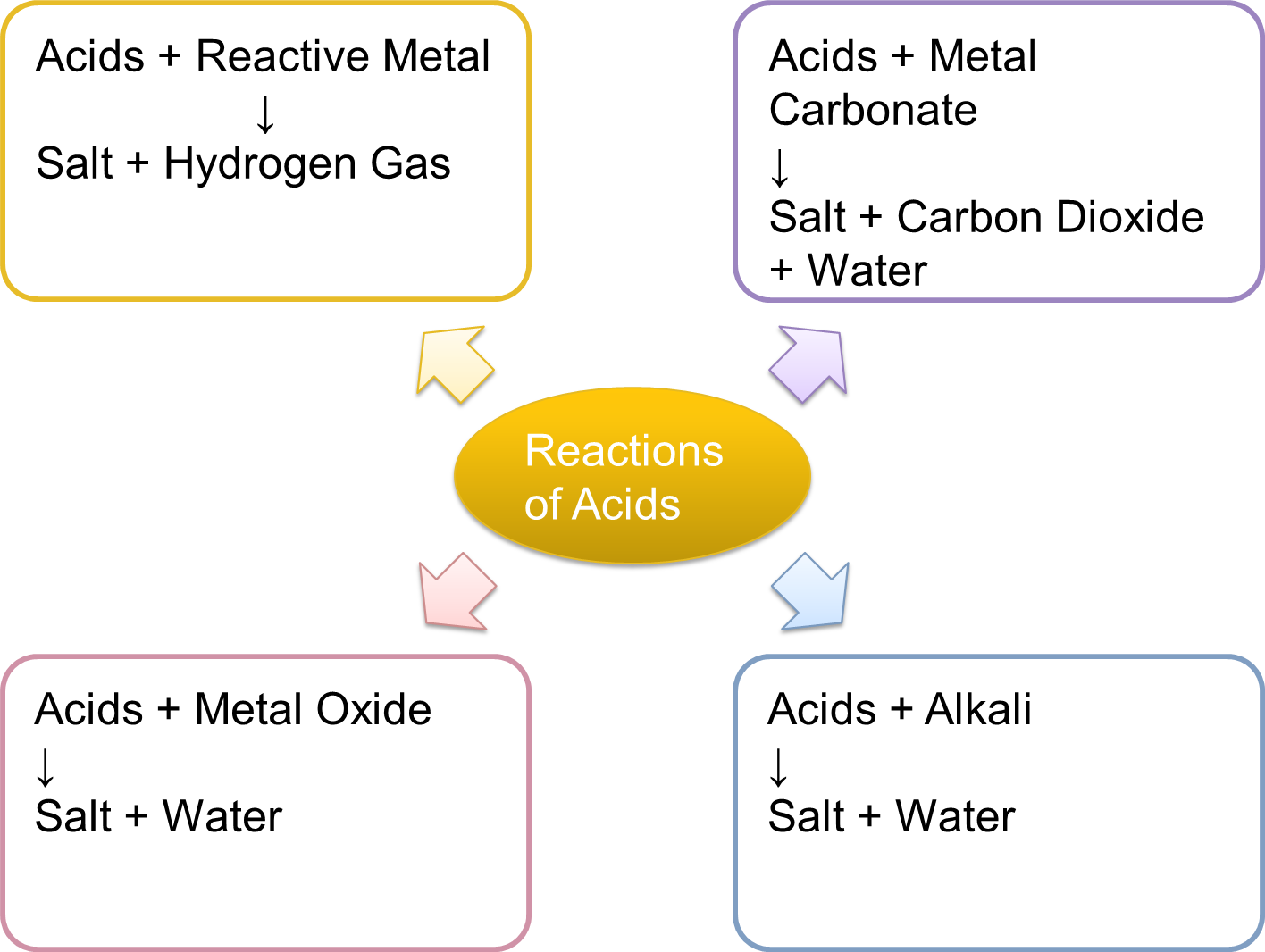
The Reactions of Acids
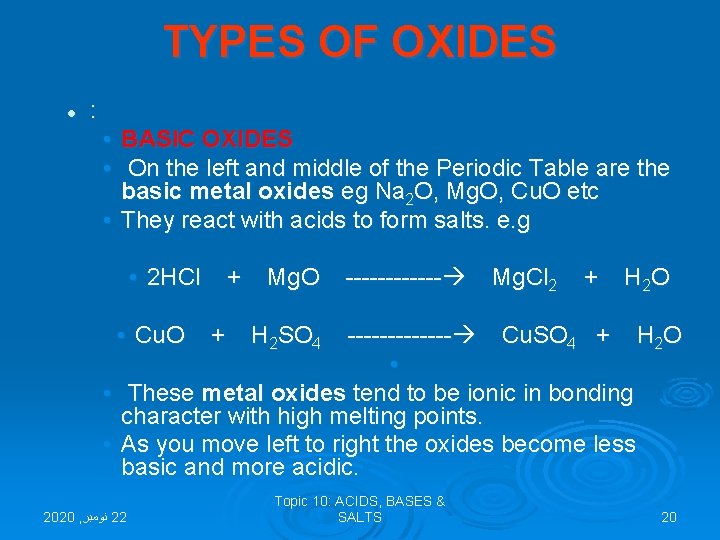
ACIDS BASES SALTS T E R M S
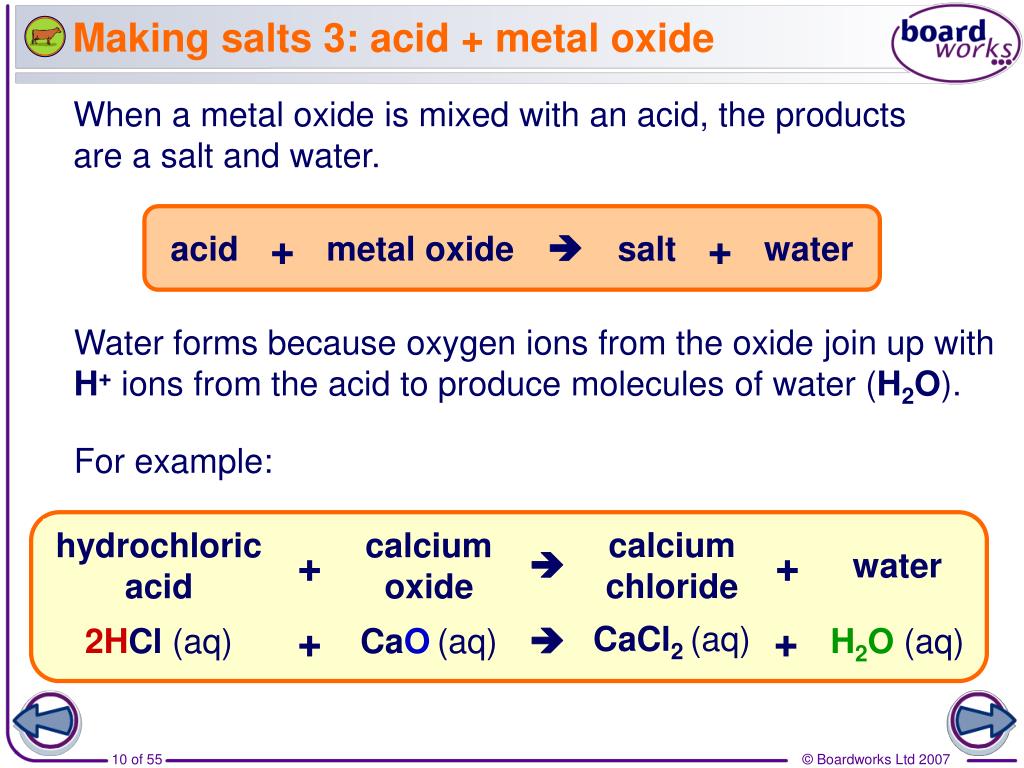
PPT How are salts made and named? PowerPoint Presentation, free download ID5348558
Chemical Properties of Acids SPM Chemistry
PPT Chemical Equations and Reactions PowerPoint Presentation, free download ID9254120

Why does copper oxide not dissolve in water

Making Salts From Acids & Metal Carbonates (GCSE Chemistry) YouTube
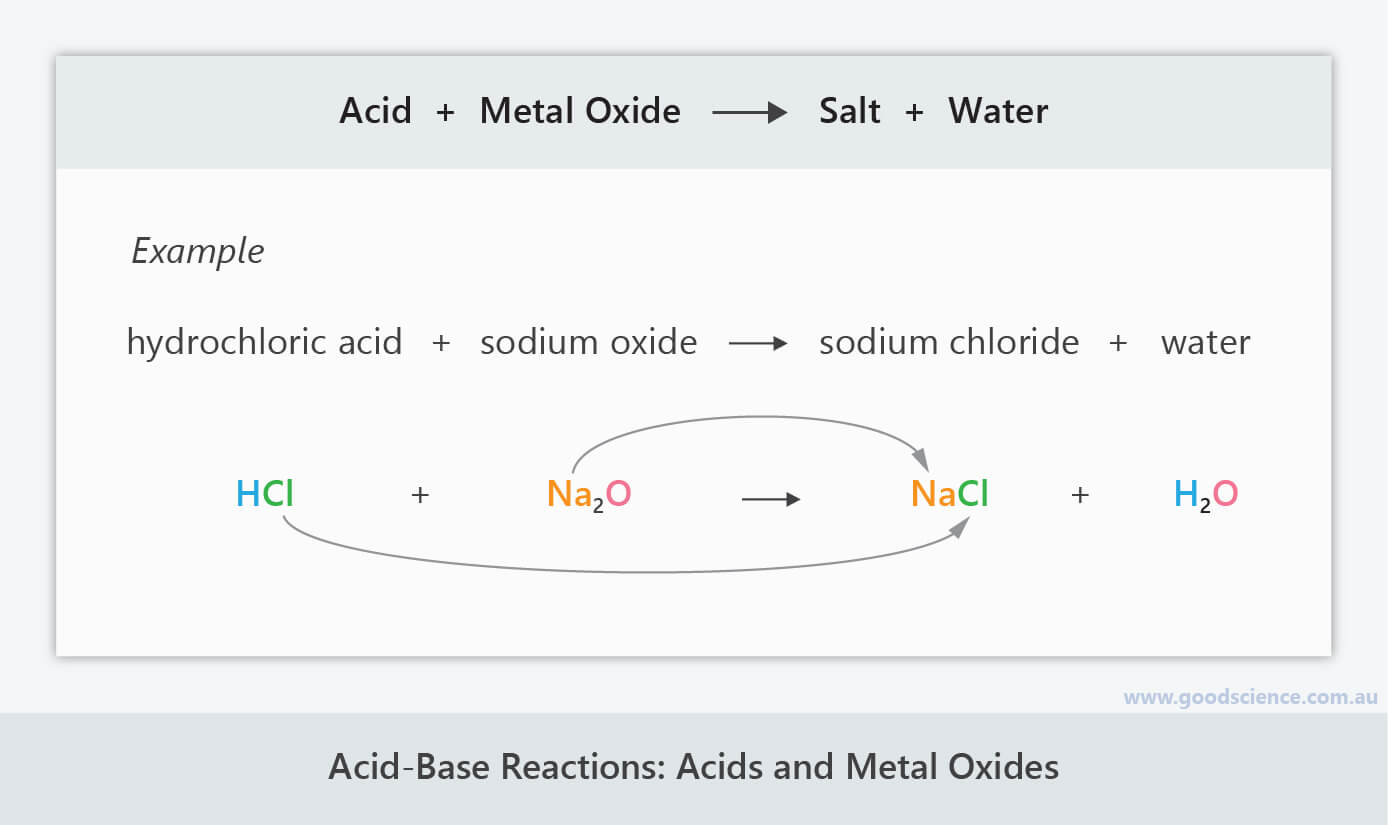
AcidBase Reactions Good Science

How acids react with metallic oxides CBSE Class 10 Chemistry Notes YouTube

Water, Acids and Bases Mr. Halfen Nov ppt download
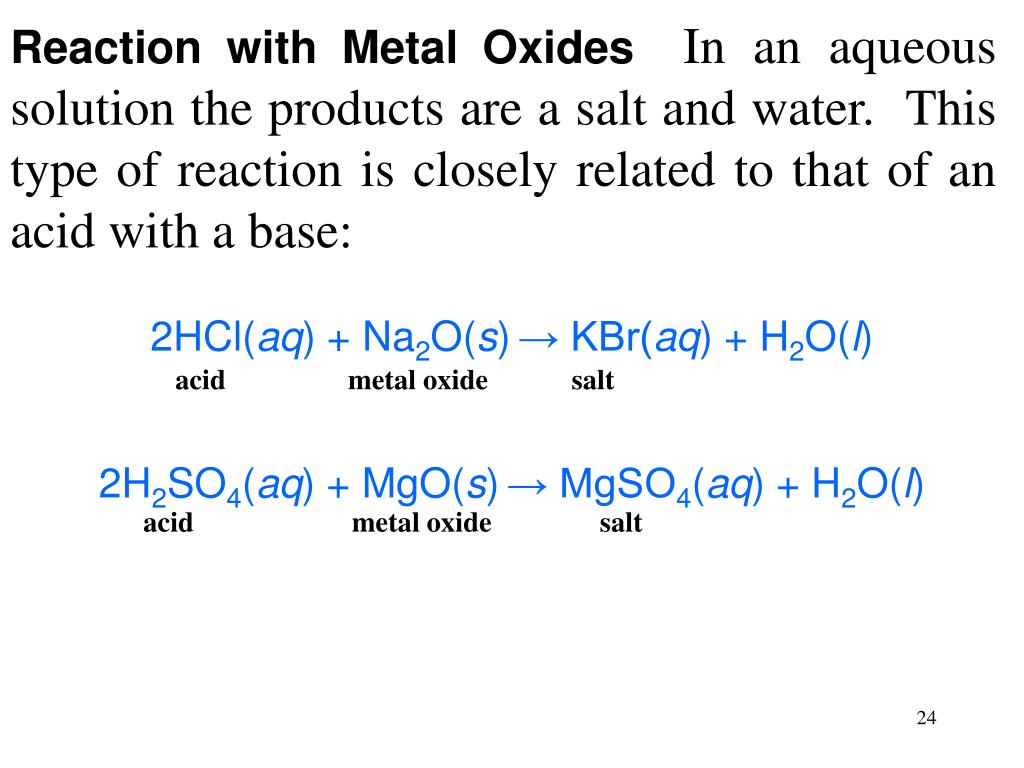
PPT Acids, Bases and Salts PowerPoint Presentation, free download ID6584954
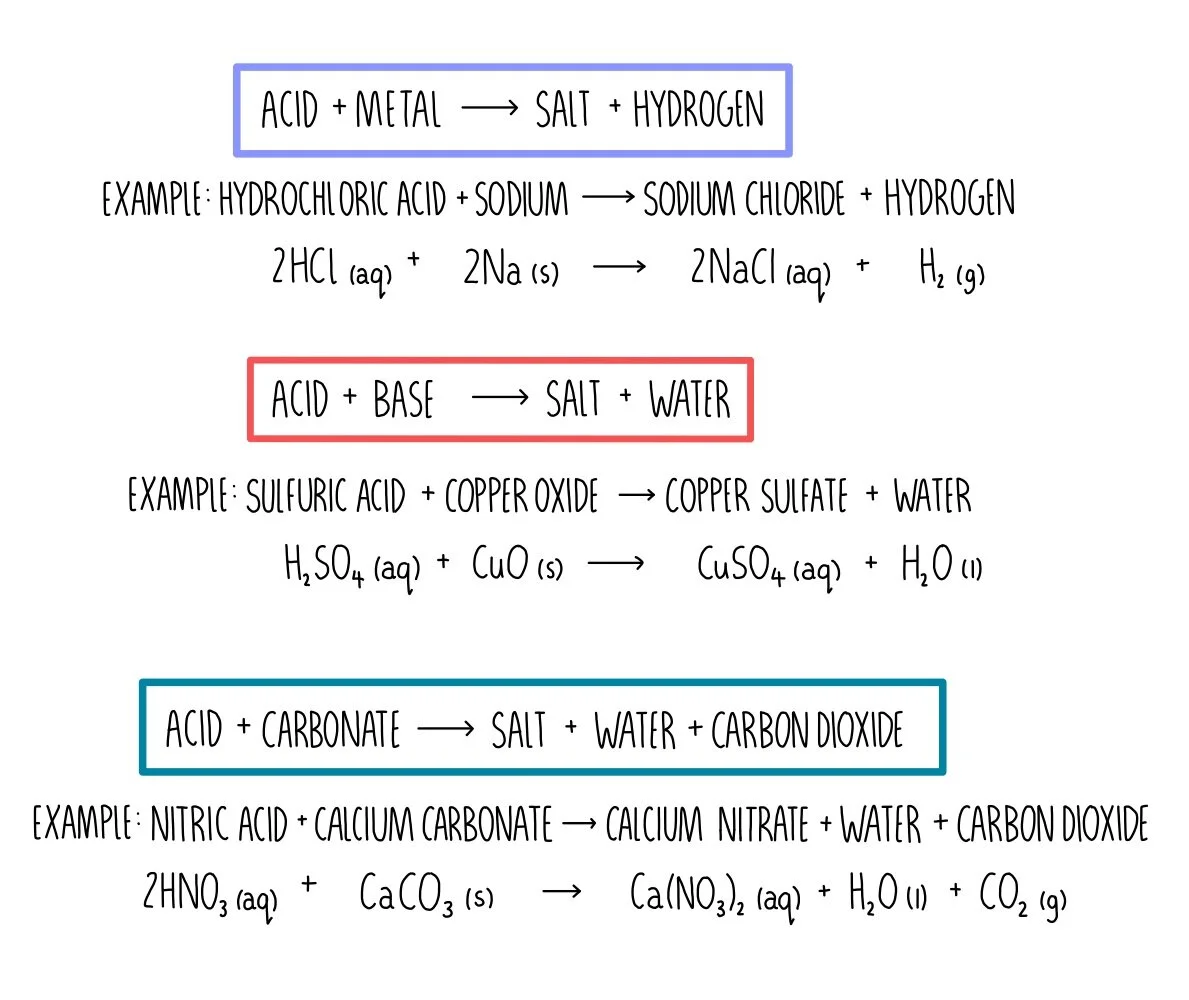
Acids, Bases and Salt Preparations (GCSE) — the science hive
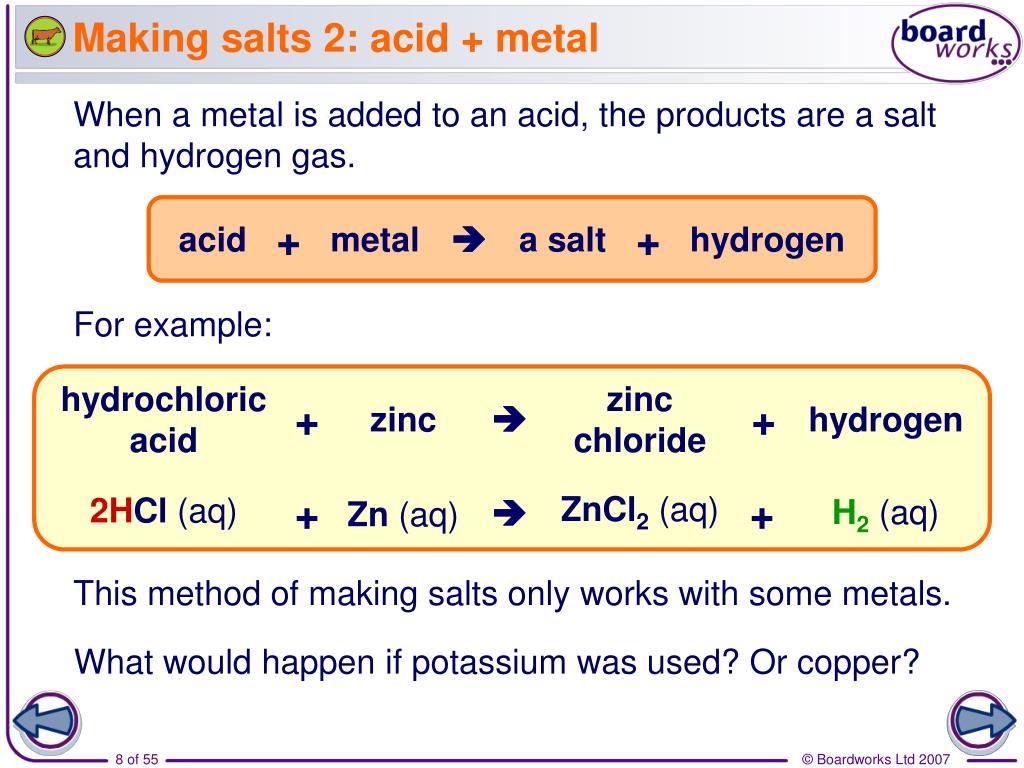
PPT How are salts made and named? PowerPoint Presentation, free download ID5348558

Acids Bases Salts Lesson 10 Reaction of Metallic Oxide with Acids & NonMetallic oxides with

PPT Working With Chemical Reactions PowerPoint Presentation ID3661856
Nitric acid will react with a metal carbonate or bicarbonate to form a salt, CO2 and water. Now the formation of salt depends on which metal carbonate or bicarbonate we are using. For ex if nitric acid reacts with sodium carbonate, sodium nitrate, Co2 and water are the products formed. 2 comments. ( 2 votes). The imperative reduction of carbon dioxide into valuable fuels stands as a crucial step in the transition towards a more sustainable energy system. Perovskite oxides, with their high compositional and property adjustability, emerge as promising catalysts for this purpose, whether employed independently or as a supporting matrix for other active metals. In this study, an A-site-deficient La0.